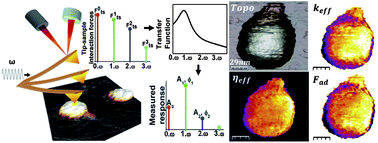
Understanding the relationships between viral material properties (stiffness, strength, charge density, adhesion, hydration, viscosity, etc.), structure (protein sub-units, genome, surface receptors, appendages), and functions (self-assembly, stability, disassembly, infection) is of significant importance in physical virology and nanomedicine. Conventional Atomic Force Microscopy (AFM) methods have measured a single physical property such as the stiffness of the entire virus from nano-indentation at a few points which severely limits the study of structure–property–function relationships. We present an in vitro dynamic AFM technique operating in the intermittent contact regime which synthesizes anharmonic Lorentz-force excited AFM cantilevers to map quantitatively at nanometer resolution the local electro-mechanical force gradient, adhesion, and hydration layer viscosity within individual ϕ29 virions. Furthermore, the changes in material properties over the entire ϕ29 virion provoked by the local disruption of its shell are studied, providing evidence of bacteriophage depressurization. The technique significantly generalizes recent multi-harmonic theory (A. Raman, et al., Nat. Nanotechnol., 2011, 6, 809–814) and enables high-resolution in vitro quantitative mapping of multiple material properties within weakly bonded viruses and nanoparticles with complex structure that otherwise cannot be observed using standard AFM techniques.

 Please wait while we load your content...
Please wait while we load your content...