Solution equilibrium behind the room-temperature synthesis of nanocrystalline titanium dioxide†
Abstract
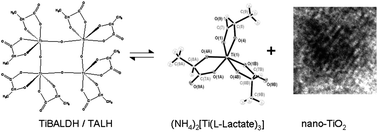
Formation of nanocrystalline and monodisperse TiO2 from a water soluble and stable precursor, ammonium oxo-lactato-titanate, (NH4)8Ti4O4(Lactate)8·4H2O, often referred to as TiBALDH or TALH, is demonstrated to be due to a coordination equilibrium. This compound, individual in the solid state, exists in solution in equilibrium with ammonium tris-lactato-titanate, (NH4)2Ti(Lactate)3 and uniform crystalline TiO2 nanoparticles (anatase) stabilized by surface-capping with lactate ligands. This equilibrium can be shifted towards nano-TiO2via application of a less polar solvent like methanol or ethanol, dilution of the solution, introduction of salts or raising the temperature, and reverted on addition of polar and strongly solvating media such as dimethyl sulfoxide, according to NMR. Aggregation and precipitation of the particles were followed by DLS and could be achieved by a decrease in their surface charge by adsorption of strongly hydrogen-bonding cations, e.g. in solutions of ammonia, ethanolamine or amino acid arginine or by addition of ethanol. The observed equilibrium may be involved in formation of nano-titania on the surface of plant roots exerting chelating organic carboxylate ligands and thus potentially influencing plant interactions.

 Please wait while we load your content...
Please wait while we load your content...