17O NMR studies of boronic acids and their derivatives†
Abstract
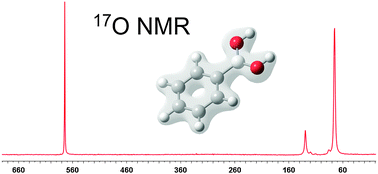
The group of 155 substituted phenylboronic acids and their derivatives: esters, boroxines and benzoxaboroles, were investigated by 17O NMR spectroscopy. The influence of substituents of phenylboronic acids on the 17O chemical shift was evaluated. For the compounds with substituents at the para position, excellent correlation with the Hammett constant was obtained. For the ortho-substituted compounds use of the Charton equation gave a good correlation of results. Surprisingly, meta-substituted compounds show none of the above-mentioned correlations. A series of boronic esters of alcohols, diols and hydroxyacids were synthesized. The influence of the structure of parent hydroxy compounds on the 17O chemical shift was discussed. Selected benzoxaboroles and boroxines were also investigated, and their chemical shifts were compared with those of boronic acids. 17O NMR spectroscopy was also used to investigate the equilibria in solution. Equilibrium of the reaction of selected boronic acids with hydroxyl anions in acetonitrile was determined by 17O and 11B titration experiments. Monomer–dimer equilibria were also studied. The experimental chemical shifts were compared with the results of theoretical calculations. Very good correlations were obtained for the ortho- and para-substituents, but not for the meta ones.

 Please wait while we load your content...
Please wait while we load your content...