Selective detection of cyanide by a polyfluorene-based organoboron fluorescent chemodosimeter
Abstract
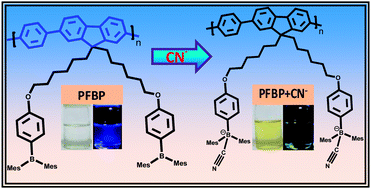
Organoboron-based polymeric fluorescent probes have been designed and synthesized for the sensitive and selective detection of toxic cyanide ions by a fluorescence “turn off” mechanism. Bis-(bromohexyl)-polyfluorenes have been synthesized by Pd catalyzed Suzuki cross-coupling polymerization. Its treatment with (Mes)2BF achieved organoboron-appended polyfluorene (PFBP). PFBP that is soluble in common organic solvents shows bright blue luminescence under UV-irradiation. The substantial quenching of the luminescence of a PFBP solution in the presence of toxic cyanide ions (CN−) has been observed. This is due to the formation of strong covalent bonds between the cyanide ions and trivalent boron centres of the side chains of the PFBP, which is further supported by NMR studies. The polyfluorene backbone of the PFBP acts as the fluorophore, and the boron centre as the cyanide acceptor. This novel type of polymeric material will find a potential application in sensor technology, as the detection limit is very low at ∼0.5 μM.

 Please wait while we load your content...
Please wait while we load your content...