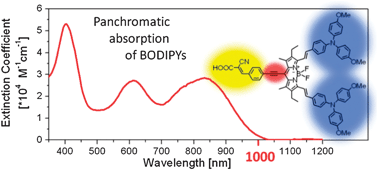
We report the synthesis of meso-ethynylphenyl BODIPYs and compare their properties with the corresponding meso-phenyl derivatives. Both types of BODIPYs carry a 2-cyano-3-acrylic acid anchoring moiety and either methyl groups or 4,4′-dimethoxytriphenylamine (MeOTPA) donor groups at positions 3 and 5. All compounds were characterized by NMR, UV/vis and cyclic voltammetry. The MeOTPA-substituted BODIPYs show an excellent panchromatic absorption with high molar extinction coefficients over the whole UV/vis range up to the near-IR region. The most impressive absorption was exhibited by the MeOTPA-substituted meso-ethynylphenyl BODIPY which strongly absorbs up to 1030 nm. By cyclic voltammetry measurements, all compounds were identified to be electrochemically stable in solution. Further, it was observed that the value of the LUMO level can be tuned by the meso-substituent. The HOMO level is determined by the donor substituents (−5.41 ± 0.03 eV and −4.84 ± 0.01 eV for BODIPYs with methyl groups and MeOTPA donor groups, respectively). These findings were further supported by DFT calculations. To evaluate the potential of the BODIPYs as sensitizers, the incident photon-to-current conversion efficiencies of solid-state dye-sensitized solar cells were measured. The photoaction spectra clearly show that the BODIPYs contribute to the photocurrent generation over their entire absorption region.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...