Lewis acidity and sugar receptor activity of 3-amino-substituted benzoxaboroles and their ortho-aminomethylphenylboronic acid analogues†
Abstract
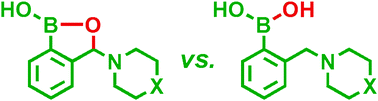
The pKas as well as apparent binding constants of several 3-amino-substituted benzoxaboroles with alizarin red S (ARS) and some biologically important sugars have been evaluated and compared with that of the parent aminomethylphenylboronic acids. The investigated benzoxaboroles reveal lower acidity than the corresponding

 Please wait while we load your content...
Please wait while we load your content...