The hexacarbonyldicobalt derivative of aspirin acts as a CO-releasing NSAID on malignant mesothelioma cells†
Abstract
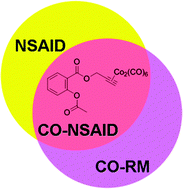
The antiproliferative activity of the aspirin derivative [2-acetoxy-(2-propynyl)benzoate]hexacarbonyldicobalt (Co-ASS) and its analogue hexacarbonyl[μ-(2-ethylphenyl)methanol]dicobalt (Co-EPM) was investigated on malignant pleural mesothelioma (MPM) cell lines, having an epithelioid or a sarcomatoid phenotype. In sarcomatoid cell lines Co-ASS was more potent than Co-EPM and the prototypal metallo-drug cisplatin, and induced cell death through the intrinsic apoptotic pathway, associated with a strong NF-κB inhibition. In contrast, both Co-ASS and Co-EPM showed only a modest cytostatic activity against epithelioid MPM cells. Co-EPM induced an increase of senescent cells, while Co-ASS did not; the different outcomes were traced back to the organic (aspirin-like) portion of the molecule. Both Co-EPM and Co-ASS significantly reduced reactive oxygen/nitrogen species (ROS/RNS), and in turn nitrites, suggesting that the hexacarbonyldicobalt moiety may deliver CO within the cell, acting as a CO-releasing molecule (CO-RM). In perspective, Co-ASS would be better considered as a CO-NSAID agent (a CO-releasing molecule retaining the NSAID properties similar to NO- and H2S-NSAIDs) than as an antitumor drug candidate.

 Please wait while we load your content...
Please wait while we load your content...