*
Corresponding authors
a
Barcelona Science Park, Baldiri Reixac 10-12, 08028 Barcelona, Spain
E-mail:
rlavilla@pcb.ub.es
Tel: +34-934037106
b
Institute for Research in Biomedicine, Barcelona Science Park, Baldiri Reixac 10-12, 08028 Barcelona, Spain
c
Department of Organic Chemistry, Faculty of Chemistry, University of Barcelona, Martí I Franqués 1-11, 08028 Barcelona, Spain
d
CIBER-BBN, Networking Centre on Bioengineering Biomaterials and Nanomedicine, Barcelona Science Park, Baldiri Reixac 10, 08028 Barcelona, Spain
e
Department of Patology and Experimental Therapeutics, Faculty of Medicine, University of Barcelona, Feixa Llarga s/n, Pavelló de Govern. 08907 L'Hospitalet, Barcelona, Spain
f
School of Chemistry, University of KwaZulu-Natal, 4001-Durban, South Africa
g
Laboratory of Organic Chemistry, Faculty of Pharmacy, University of Barcelona, Avda. Joan XXII s.n., 08028 Barcelona, Spain
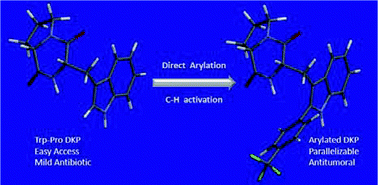
 Please wait while we load your content...
Please wait while we load your content...