Identification of 4,6-diaryl-1,4-dihydropyridines as a new class of neuroprotective agents†
Abstract
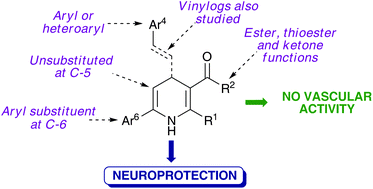
A library of 4,6-diaryl-1,4-dihydropyridines was synthesized using a CAN-catalyzed, Hantzsch-related three component reaction starting from ammonium acetate, β-dicarbonyl compounds and a variety of α,β-unsaturated ketones including chalcones, their vinylogs and heteroanalogues. These compounds lack the structural features needed for vascular activity and were found to prevent calcium overload and behave as neuroprotective agents. One of the compounds, bearing a 2-thienyl substituent at C-4, showed the highest neuroprotective activity and was also a moderate antioxidant, being a good lead compound for further studies in this area.
 Please wait while we load your content...
Please wait while we load your content...