6-Alkyl-, 6-aryl- or 6-hetaryl-7-deazapurine ribonucleosides as inhibitors of human or MTB adenosine kinase and potential antimycobacterial agents†
Abstract
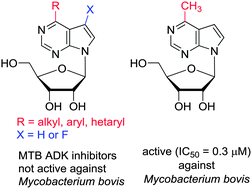
Title 6-alkyl-, 6-aryl- and 6-hetaryl-7-deazapurine ribonucleosides previously known as nanomolar cytostatics were found to be potent inhibitors of either human or mycobacterial (MTB) adenosine kinase (ADK). Several new derivatives bearing bulky substituents at position 6 were non-cytotoxic but selectively inhibited MTB ADK. However, most of the nucleosides (ADK inhibitors) as well as their octadecylphosphate prodrugs were inactive in the whole cell assay of inhibition of Mycobacterium bovis growth. 6-Methyl-7-deazapurine ribonucleoside was found to be a potent antimycobacterial agent.
 Please wait while we load your content...
Please wait while we load your content...