Flavans from Desmos cochinchinensis as potent aromatase inhibitors†
Abstract
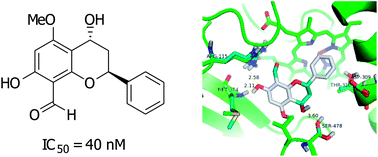
Flavans from the roots of Desmos cochinchinensis exhibited potent aromatase inhibitory activity at nanomolar levels, and could be leads for the development of anti-aromatase drugs. In addition, these aromatase inhibitors did not show pronounced cytotoxic activity. Flavans exert their inhibitory activity through binding, as revealed by molecular docking studies, with aromatase at Arg115, Met374, and Leu477; the C-7 hydroxyl (or methoxyl) forms hydrogen bonds with Met374 and Arg115 of aromatase.
 Please wait while we load your content...
Please wait while we load your content...