Polyhydroxylated pyrrolidine and 2-oxapyrrolizidine as glycosidase inhibitors†
Abstract
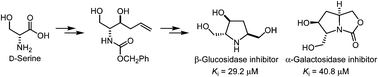
Using D-serine as a chiral precursor, a polyhydroxylated pyrrolidine (1), its derivatives bearing carboxylate, phosphate and phosphonate groups (2–4) and an oxapyrrolizidine (5) were synthesized. The pyrrolidine ring was formed by intramolecular amino-mercuration. The bicyclic scaffold of oxapyrrolizidine was further constructed by an intramolecular attack of the carbamate group on the iodomethyl group. Compounds 1 and 5 were found to inhibit β-glucosidase and α-galactosidase, respectively, in a competitive manner, whereas compounds 2, 3 and 4 did not produce significant inhibition against glycosidases.

 Please wait while we load your content...
Please wait while we load your content...