BH3 helix-derived biophotonic nanoswitches regulate cytochrome c release in permeabilised cells†
Abstract
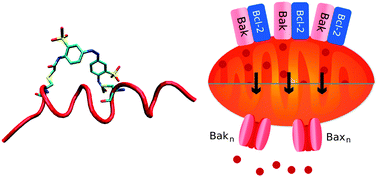
Dynamic physical interactions between
* Corresponding authors
a
School of Chemistry, Cardiff University, Main Building, Park Place, Cardiff CF10 3AT, UK
E-mail:
allemannrk@cardiff.ac.uk
Fax: +44 (0)29 208 74030
Tel: +44 (0)29 208 79014
b Cardiff Catalysis Institute, Cardiff University, Main Building, Park Place, Cardiff CF10 3AT, UK
c Institute of Cancer and Genetics, School of Medicine, Cardiff University, Heath Park, Cardiff CF14 4XN, UK
d Cardiff School of Pharmacy and Pharmaceutical Sciences, Cardiff University, Redwood Building, King Edward VII Avenue, Cardiff CF10 3NB, UK
Dynamic physical interactions between

 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
R. J. Mart, R. J. Errington, C. L. Watkins, S. C. Chappell, M. Wiltshire, A. T. Jones, P. J. Smith and R. K. Allemann, Mol. BioSyst., 2013, 9, 2597 DOI: 10.1039/C3MB70246D
This article is licensed under a Creative Commons Attribution 3.0 Unported Licence. You can use material from this article in other publications without requesting further permissions from the RSC, provided that the correct acknowledgement is given.
Read more about how to correctly acknowledge RSC content.
 Fetching data from CrossRef.
Fetching data from CrossRef.
This may take some time to load.
Loading related content
