One-pot combination of enzyme and Pd nanoparticle catalysis for the synthesis of enantiomerically pure 1,2-amino alcohols†
Abstract
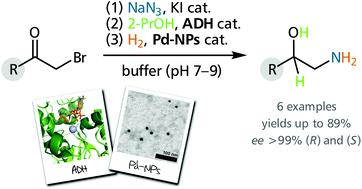
One-pot combinations of sequential catalytic reactions can offer practical and ecological advantages over classical multi-step synthesis schemes. In this context, the integration of enzymatic and chemo-catalytic transformations holds particular potential for efficient and selective reaction sequences that would not be possible using either method alone. Here, we report the one-pot combination of alcohol dehydrogenase-catalysed asymmetric reduction of 2-azido ketones and Pd nanoparticle-catalysed hydrogenation of the resulting azido alcohols, which gives access to both enantiomers of aromatic 1,2-amino alcohols in high yields and excellent optical purity (ee >99%). Furthermore, we demonstrate the incorporation of an upstream azidolysis and a downstream acylation step into the one-pot system, thus establishing a highly integrated synthesis of the antiviral natural product (S)-tembamide in 73% yield (ee >99%) over 4 steps. Avoiding the purification and isolation of intermediates in this synthetic sequence leads to an unprecedentedly low ecological footprint, as quantified by the E-factor and solvent demand.

 Please wait while we load your content...
Please wait while we load your content...