A DFT study of furan hydrogenation and ring opening on Pd(111)
Abstract
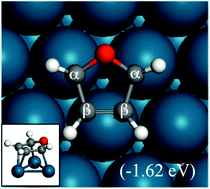
The reaction energies and the corresponding energy barriers of hydrogenation and ring opening of furan on Pd(111) for the formation of tetrahydrofuran (THF), 1-butanol and small hydrocarbons were studied using density functional theory (DFT). THF forms via sequential hydrogenation of carbon atoms of the furan ring in the order of α-carbon, adjacent β-carbon, second β-carbon, and the remaining α-carbon. Upon hydrogenation of the α-carbon of furan, ring opening becomes facile. Thus, hydrofuran (HF) is a reactive intermediate in both hydrogenation and ring opening. The fate of HF determines the selectivity of the overall reaction. A simple kinetic analysis indicates that coverage effects are important and the hydrogen partial pressure is a key factor in controlling selectivity. Dihydrofuran (DHF) was found to be a stable intermediate, consistent with experimental findings. Once DHF is formed, ring opening is not favored due to the high energy barriers of ring opening of DHF, trihydrofuran (TriHF) and THF. 1-Butanol is a thermodynamically favored product, while THF is kinetically preferred. Our theoretical work agrees well with experimental observations that 1-butanol is a major product at high temperatures whereas THF is a major product at low temperatures. Insights gained into selectivity toward ring hydrogenation and ring opening can assist future studies in catalyst selection.
- This article is part of the themed collection: Conversion of biomass with heterogeneous catalysts

 Please wait while we load your content...
Please wait while we load your content...