Enzymatic cleavage of lignin β-O-4 aryl ether bonds via net internal hydrogen transfer†
Abstract
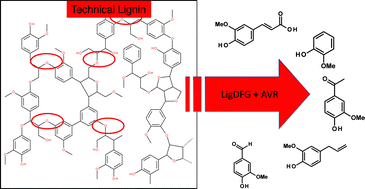
The current greening of chemical production processes going along with a rising interest for the utilization of biogenic feedstocks recently revived the research to find new ways for the degradation of the complex lignin-backbone by means of biocatalysis and combined chemo-enzymatic catalysis. Lignin, which accumulates in 50 million t/a, is regarded as a potential substitute for phenolic and other aromatic, oil-based chemicals in the upcoming post oil age. The cleavage of the β-O-4-aryl ether linkage is the most favoured, since it accounts for approximately 50% of all ether linkages in lignin. This enzymatic cleavage was proposed to be a part of the lignin catabolism in the proteobacterium Sphingobium sp. SYK6. Three enzymes, LigD, a Cα-dehydrogenase, LigF, a β-etherase and LigG, a glutathione lyase, are supposed to be involved in lignin degradation. We cloned and recombinantly expressed these genes in E. coli and determined their pH and temperature optima on the lignin model substrate 1-(4-hydroxy-3-methoxyphenyl)-2-(2-methoxyphenoxy)-1,3-propanediol 1. Using an NAD+ dependent glutathione reductase from Allochromatium vinosum (AVR) we established an efficient way to regenerate the co-substrates NAD+ and glutathione allowing for a self-sufficient balanced enzymatic cascade with net internal hydrogen transfer (hydrogen borrowing). We showed the capability of this enzyme system to release lignin monomers from complex lignin structures coming from differently prepared real lignin substrates. This novel enzyme system could become a useful tool to release lignin monomers from complex lignin structures.

 Please wait while we load your content...
Please wait while we load your content...