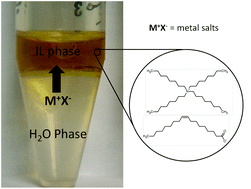
In this paper new extractants, that have the potential to be sustainably regenerated, are proposed for metal removal from aqueous phases. These extractants are a novel class of ionic liquids (ILs) with unsaturated fatty acids as anions (oleate and linoleate). The advantages of using these ILs are their simple synthesis, their sustainability (fatty acids anions are renewable), their biocompatibility and their non-toxicity. This makes these ILs “simpler and greener” compared to other solvents used for metal extraction. The newly synthesized ILs were evaluated for extraction of Li, Na, K, Mn(II), Fe(II), and Zn(II) chlorides from aqueous solutions. No or negligible extraction efficiencies were observed for the alkali metal salts, but excellent extraction (>99%) efficiencies were obtained for the period IV transition metal salts.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...