Microwave-assisted, sequential four-component synthesis of polysubstituted 5,6-dihydroquinazolinones from acyclic precursors and a mild, halogenation-initiated method for their aromatization under focused microwave irradiation†
Abstract
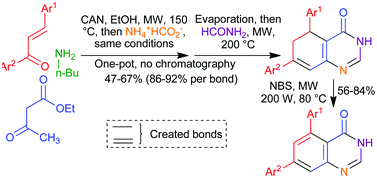
A one-pot, microwave-assisted protocol has been developed for the synthesis of 5,6-dihydroquinazolinones that incorporate structural fragments from

 Please wait while we load your content...
Please wait while we load your content...