Lewis acid–surfactant-combined catalyzed synthesis of 4-aminocyclopentenones from glycals in water†
Abstract
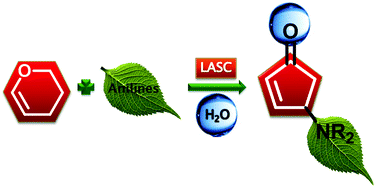
4-Aminocyclopentenones were synthesized from readily available glycals and secondary anilines, with the aid of a Lewis acid–surfactant-combined catalyst. The reactions proceeded via a 4π conrotatory electrocyclization, affording the corresponding 4-aminocyclopentenones in good yields with excellent diastereoselectivities.

 Please wait while we load your content...
Please wait while we load your content...