“All water chemistry” for a concise total synthesis of the novel class anti-anginal drug (RS), (R), and (S)-ranolazine†
Abstract
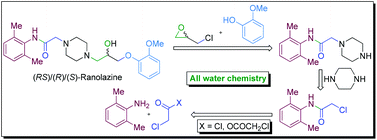
A novel strategy of ‘all water chemistry’ is reported for a concise total synthesis of the novel class anti-anginal drug ranolazine in its racemic (RS) and enantiopure [(R) and (S)] forms. The reactions at the crucial stages of the synthesis are promoted by water and led to the development of new water-assisted chemistries for (i) catalyst/base-free N-acylation of amine with acyl anhydride, (ii) base-free N-acylation of amine with acyl chloride, (iii) catalyst/base-free one-pot tandem N-alkylation and N-Boc deprotection, and (iv) base-free selective mono-alkylation of diamine (e.g., piperazine). The distinct advantages in performing the reactions in water have been demonstrated by performing the respective reactions in organic solvents that led to inferior results and the beneficial effect of water is attributed to the synergistic electrophile and nucleophile dual activation role of water. The new ‘all water’ strategy offers two green processes for the total synthesis of ranolazine in two and three steps with 77 and 69% overall yields, respectively, and which are devoid of the formation of the impurities that are generally associated with the preparation of ranolazine following the reported processes.

 Please wait while we load your content...
Please wait while we load your content...