Direct aerobic oxidation of 2-benzylpyridines in a gas–liquid continuous-flow regime using propylene carbonate as a solvent†
Abstract
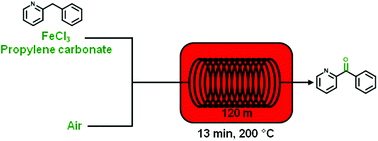
The use of high-temperature/pressure gas–liquid continuous flow conditions dramatically enhances the iron-catalyzed aerobic
- This article is part of the themed collection: Green Solvents

 Please wait while we load your content...
Please wait while we load your content...