Molten air – a new, highest energy class of rechargeable batteries†
Abstract
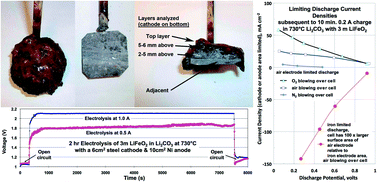
This study introduces the principles of a new class of high-energy batteries and their fundamental chemistry is demonstrated. These molten air batteries use air, a molten electrolyte, are quasi-reversible (rechargeable), have the capability for multiple electrons stored per molecule, and have the highest intrinsic electric energy storage capacities. Here we show three examples of the new battery's electron transfer chemistry. These are the metal, carbon and VB2 molten air batteries with respective intrinsic volumetric energy capacities of 10 000 (for Fe to Fe(III)), 19 000 (C to CO32−) and 27 000 W h l−1 (VB2 to B2O3 + V2O5), compared to 6200 W h l−1 for the lithium air battery. Higher energy capacity, cost effective batteries are needed for a range of electronic, transportation and greenhouse gas reduction power generation devices. Needed greenhouse gas battery reduction applications include overcoming the battery driven “range anxiety” of electric vehicles, and increased capacity energy storage for the electric grid.

 Please wait while we load your content...
Please wait while we load your content...