Direct observation of the reduction of carbon dioxide by rhenium bipyridine catalysts†
Abstract
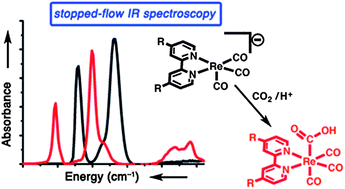
In order to further efforts in synthesis and catalysis, the mechanisms of catalysts must be completely understood. The Re(bpy)(CO)3Cl molecular catalysts are some of the most robust and well-characterized CO2 reduction catalysts known to date. Stopped-flow infrared spectroscopy is reported as a technique for studying the kinetics and mechanisms of the reactions of catalytically-relevant [Re(bpy-R)(CO)3]− anions (R = tBu or H) with CO2/H+. [Re(bpy-tBu)(CO)3]− reacts approximately ten times faster with CO2 than does [Re(bpy)(CO)3]−. These reactions occur via a direct two-electron oxidative addition of CO2 to the metal center and result in the formation of an intermediate CO2 reduction product, Re(bpy-R)(CO)3(CO2H). This is the first in situ identification of this key intermediate. Evidence for this Re–CO2H species includes isotopic labeling studies, stopped-flow experiments of the kinetics of its formation in the presence of proton sources, comparison with genuine Re(bpy)(CO)3(CO2H), and DFT calculations.

 Please wait while we load your content...
Please wait while we load your content...