Current density dependence of peroxide formation in the Li–O2 battery and its effect on charge†
Abstract
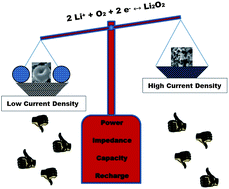
We report a significant difference in the growth mechanism of Li2O2 in Li–O2 batteries for toroidal and thin-film morphologies which is dependent on the current rate that governs the electrochemical pathway. Evidence from diffraction, electrochemical,
- This article is part of the themed collection: Post lithium ion batteries

 Please wait while we load your content...
Please wait while we load your content...