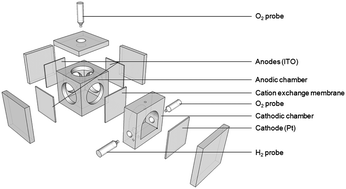
Microbial electrolysis cells (MECs) represent an emerging technology that uses heterotrophic microbes to convert organic substrates into fuel products, such as hydrogen gas (H2). The recent development of biophotovoltaic cells (BPVs), which use autotrophic microbes to produce electricity with only light as a substrate, raises the possibility of exploiting similar systems to harness photosynthesis to drive the production of H2. In the current study we explore the capacity of the cyanobacterium Synechocystis sp. PCC 6803 to generate electrons by oxygenic photosynthesis and facilitate H2 production in a two-chamber bio-photoelectrolysis cell (BPE) system using the electron mediator potassium ferricyanide ([Fe(CN)6]3−). The performance of a wild-type and mutant strain lacking all three respiratory terminal oxidase activities (rto) was compared under low or high salt conditions. The rto mutant showed a decrease in maximum photosynthetic rates under low salt (60% lower Pmax than wild-type) but significantly increased rates under high salt, comparable to wild-type levels. Remarkably, rto demonstrated a 3-fold increase in (Fe[CN]6)3− reduction rates in the light under both low and high salt compared to the wild-type. Yields of H2 and efficiency parameters were similar between wild-type and rto, and highest under high salt conditions, resulting in a maximum rate of H2 production of 2.23 ± 0.22 ml H2 l−1 h−1 (0.68 ± 0.11 mmol H2 [mol Chl]−1 s−1). H2 production rates were dependent on the application of a bias-potential, but all voltages used were significantly less than that required for water electrolysis. These results clearly show that production of H2 using cyanobacteria is feasible without the need to inhibit photosynthetic O2 evolution. Optimising the balance between the rates of microbial-facilitated mediator reduction with H2 production may lead to long-term sustainable H2 yields.
This article is Open Access
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...