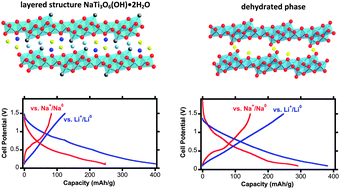
The electrochemical properties of materials derived from NaTi3O6(OH)·2H2O have been investigated for the first time. The parent compound has a corrugated layered structure consisting of {Ti6O14}4− units with hydrated sodium cations and protons in the interlayer spaces. Upon heating to 600 °C, water is removed irreversibly, the interlayer distances become smaller, and connecting bonds between the octahedral layers form. It was found that this material can reversibly intercalate both lithium and sodium. The initial specific discharge capacities, as measured in half-cells, varied with the state of hydration and the nature of the counter electrode (Na or Li). The electrochemical potential showed a non-linear sloping dependence with degree of intercalation, indicative of a solid-solution mechanism of intercalation. The process was centered at a low average potential of about 0.3 V vs. Na or Li, the lowest ever reported for titanate-based Li hosts. The higher density and potential for higher rate capability of this compound, in comparison to carbonaceous materials with similar voltage and reversible capacities, make a compelling case for its development as an anode material, for both lithium and sodium ion batteries.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...