Aqueous dye-sensitized solar cell electrolytes based on the cobalt(ii)/(iii) tris(bipyridine) redox couple†
Abstract
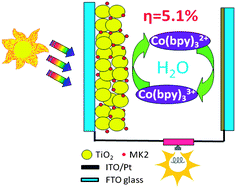
In this study, we report the first application of a cobalt(II)/(III) tris(2,2′-bipyridine) based aqueous electrolyte in the fabrication of

 Please wait while we load your content...
Please wait while we load your content...