Formation of a dicationic ruthenium benzyl complex by halide abstraction from a Grubbs-type second-generation benzylidene†
Abstract
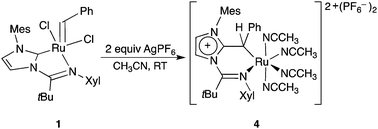
A ruthenium benzylidene complex with a neutral bidentate N-heterocyclic carbene was prepared and evaluated in ring-closing metathesis of diallyl substrates. Reaction with AgPF6 to generate a four-coordinate complex produced an unexpected dicationic ruthenium benzyl degradation product through nucleophilic attack of the NHC ligand on the benzylidene carbon.

 Please wait while we load your content...
Please wait while we load your content...