Zinc(ii), iron(ii/iii) and ruthenium(ii) complexes of o-phenylenediamine derivatives: oxidative dehydrogenation and photoluminescence†
Abstract
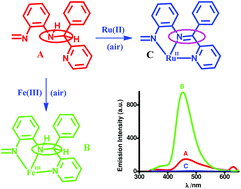
Reactions of benzoyl pyridine, o-phenylenediamine and anhydrous ZnX2 in methanol afford imine complexes [Zn(L1)X2] (X = Cl, 1; X = Br, 2) in good yields (L1 = (E)-N1-(phenyl(pyridin-2-yl)methylene)benzene-1,2-diamine). The reduction of 1 with NaBH4 affords (E)-N1-(phenyl(pyridine-2-yl)methylene)benzene-1,2-diamine (L2H). The reaction of L2H with [RuII(PPh3)3Cl2] results in the oxidative dehydrogenation to L1 generating cis-[RuII(L1)(PPh3)Cl2] (3). The reaction of L2H with salicylaldehyde affords (E)-2-(((2-((phenyl(pyridin-2-yl)methyl)amino)phenyl)imino)methyl)phenol (L3H2). The reaction of L3H2 with anhydrous FeCl3 in CH3OH affords cis-[FeIII(L3H−)Cl2] (4). Reaction of L3H2 with [RuII(PPh3)3Cl2] results in the oxidative dehydrogenation to diimine, L4H, affording trans-[RuII(L4−)(PPh3)2]+, which is isolated as trans-[RuII(L4−)(PPh3)2]PF6 (5+PF6−) (L4H = 2-((E)-(2-((E)-phenyl(pyridin-2-yl)methyleneamino)phenylimino)methyl)phenol). The reduction of L3H2 with NaBH4 produces 2-(((2-((phenyl(pyridin-2-yl)methyl)amino)phenyl)amino)methyl)phenol (L5H3). With iron(III) L5H3 undergoes oxidative dehydrogenation to L3H2 affording 4, while with [RuII(PPh3)3Cl2], L5H3 undergoes 4e + 4H+ transfer giving 5+. A fluid solution of L3H2 at 298 K exhibits an emission band at 470 nm (λex = 330 nm, τ1 = 3.70 ns) and a weaker band at 525 nm (λex = 330, 390 nm, τ1 = 1.1 ns) at higher concentrations due to molecular aggregation, which are temperature dependent. 4 is brightly emissive (λex = 330 nm, λem = 450 nm, Φ = 0.586, τ1 = 3.70 ns). Time resolved emission spectra (TRES) and lifetime measurements confirm that the lower energy absorption band of L3H2 at 390 nm, which is absent in complex 4, has a larger non-radiative rate constant (knr). The redox innocent AlIII adduct of L3H2 is fluorescent (λex = 330 nm, λem = 450 nm, τ1 = 3.70 ns). On the contrary, the cis-[FeII(L3H−)Cl2]− and cis-[Co(L3H−)Cl2]− analogues are non emissive. Density function theory (DFT) calculations, redox potentials and the near infra-red (NIR) absorption data prove that 4 is emissive due to the stable [FeIII(L3H−*)] state, while 3, 5+, cis-[FeII(L3H−)Cl2]− and cis-[Co(L3H−)Cl2]− are non-emissive due to transformations of the [MII(L*)] to [MIII(L˙−*)] states.

 Please wait while we load your content...
Please wait while we load your content...