Kinetics and mechanism of oxidation of super-reduced cobalamin and cobinamide species by thiosulfate, sulfite and dithionite†
Abstract
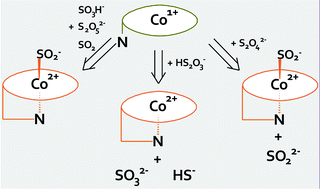
We studied the kinetics of reactions of cob(I)alamin and cob(I)inamide with thiosulfate, sulfite, and dithionite by UV-Visible (UV-Vis) and stopped-flow spectroscopy. We found that the two Co(I) species were oxidized by these sulfur-containing compounds to Co(II) forms: oxidation by excess thiosulfate leads to penta-coordinate complexes and oxidation by excess sulfite or dithionite leads to hexa-coordinate Co(II)–SO2− complexes. The net scheme involves transfer of three electrons in the case of oxidation by thiosulfate and one electron for oxidation by sulfite and dithionite. On the basis of kinetic data, the nature of the reactive oxidants was suggested, i.e., HS2O3− (for oxidation by thiosulfate), S2O52−, HSO3−, and aquated SO2 (for oxidation by sulfite), and S2O42− and SO2− (for oxidation by dithionite). No difference was observed in kinetics with cob(I)alamin or cob(I)inamide as reductants.

 Please wait while we load your content...
Please wait while we load your content...