Synthesis and characterization of homo- and heteronuclear molecular Al3+ and Th4+ species chelated by the ethylenediaminetetraacetate (edta) ligand†
Abstract
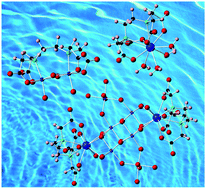
Four molecular species chelated by edta have been synthesized from aqueous solutions and characterized by ![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) with a = 9.3172(8) Å, b = 16.6099(14) Å, c = 19.5080(14) Å, α = 102.314(3)°, β = 95.615(3)°, and γ = 92.473(3)°. [Th2(H2O)2Al6(H2O)10(OH)14(edta)2]·(NO3)4(H2O)18 (Th2Al6) also crystallized in triclinic space group P
with a = 9.3172(8) Å, b = 16.6099(14) Å, c = 19.5080(14) Å, α = 102.314(3)°, β = 95.615(3)°, and γ = 92.473(3)°. [Th2(H2O)2Al6(H2O)10(OH)14(edta)2]·(NO3)4(H2O)18 (Th2Al6) also crystallized in triclinic space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) with unit cell parameters a = 10.9899(12) Å, b = 11.6107(12) Å, c = 14.3350(17) Å, α = 73.012(4)°, β = 87.411(4)°, and γ = 75.717(4)°. Small molecular species are observed in the Th1 and Al2 compounds, while the metal
with unit cell parameters a = 10.9899(12) Å, b = 11.6107(12) Å, c = 14.3350(17) Å, α = 73.012(4)°, β = 87.411(4)°, and γ = 75.717(4)°. Small molecular species are observed in the Th1 and Al2 compounds, while the metal

 Please wait while we load your content...
Please wait while we load your content...