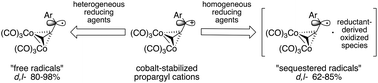
Applicability of the term “free radical” to organometallic radicals was studied by using the stereoselectivity of radical C–C bond formation as a diagnostic tool. Based on diastereoselectivity data, it was concluded that the reduction of π-bonded, Co2(CO)6-complexed propargyl cations with heterogeneous reducing agents (Zn, Mg) generates “free radicals”, while homogeneous reductants (Cp2Co, Na–Ph2CO) produce “sequestered radicals”, presumably associated with reductant-derived oxidized species. The latter are comparable in molecular volume to the requisite radical species, thus restricting the motion and conformational freedoms of converging, transition metal-complexed propargyl radicals. Diastereoselectivity of intermolecular reactions is determined to be much less sensitive toward temperature variation (Δde = 6–22%) than in intramolecular radical cyclizations (Δde = 106%).
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...