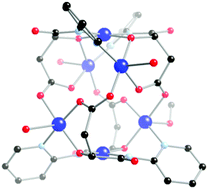
Herein we describe the synthesis, structural and magnetic characterisation of three transition metal cluster complexes that feature the polytopic ligand N-(2-pyridyl)-3-carboxypropanamide (H2222L): [Fe3IIIFe2II(HL)6(O)(H2O)3][ClO4]5·3MeCN·4H2O, 1, [Co8(HL)8(O)(OH)4(MeOH)3(H2O)]-[ClO4]3·5MeOH·2H2O, 2, and [Cu6(Lox)4(MeOH)(H2O)3]·MeOH, 3. Complex 1 is a mixed valence penta-nuclear iron cluster containing the archetypal {Fe3IIIO} triangular basic carboxylate cluster at its core, with two Fe(II) ions above and below the core coordinated to three bidentate pyridyl-amide groups. The structure of the octanuclear Co(II) complex, 2, is based upon a central Co4 square with the remaining four Co(II) centres at the ‘wing-tips’ of the complex. The cluster core is replete with bridging oxide, hydroxide and carboxylate groups. Cluster 3 contains an oxidised derivative of the ligand, Loxox, generated in situ through hydroxylation of an α-carbon atom. This hexanuclear cluster has a ‘barrel-like’ core and contains Cu(II) ions in both square planar and square-based pyramidal geometries. Bridging between Cu(II) centres is furnished by alkoxide and carboxylate groups. Magnetic studies on 1–3 reveals dominant antiferro-magnetic interactions for 1 and 2, leading to small non-zero spin ground states, while 3 shows ferro-magnetic exchange between the Cu(II) centres to give an S = 3 spin ground state.
This article is Open Access
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...