Mechanistic insights into iron catalyzed dehydrogenation of formic acid: β-hydride elimination vs. direct hydride transfer†
Abstract
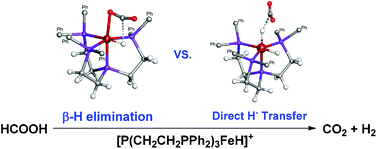
Density functional theory calculations reveal a complete reaction mechanism with detailed energy profiles and transition state structures for the dehydrogenation of formic acid catalyzed by an iron complex, [P(CH2CH2PPh2)3FeH]+. In the cationic reaction pathway, a β-hydride elimination process is confirmed to be the rate-determining step in this catalytic reaction. A potential reaction pathway starting with a direct hydride transfer from HCOO− to Fe is found to be possible, but slightly less favorable than the catalytic cycle with a β-hydride elimination step.

 Please wait while we load your content...
Please wait while we load your content...