Cuprous hydroxide in a solid form: does it exist?
Abstract
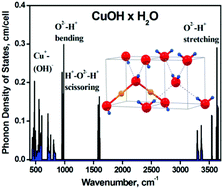
Experimental studies have been performed to obtain the unknown cuprous hydroxide compound, which has recently been predicted theoretically (P. A. Korzhavyi et. al., Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 686–689) to be metastable in a solid form. The reduction of Cu2+ with ferrous ethylenediamine tetraacetate (EDTA) results in the formation of a yellow powder precipitate whose composition corresponds to CuOH × H2O as probed by Fourier Transform Infrared Spectroscopy (FTIR) and cryogenic X-ray Photoelectron Spectroscopy (XPS). A similar compound has been found on the surface of Cu–CuH powder stored in water, as detected by XPS. The reduction of Cu2+ to Cu+ with free radicals in aqueous solutions results in a Cu2O precipitate as the final product, while the formation of the yellow cuprous hydroxide colloids may be an intermediate step. Our studies reveal that cuprous hydroxide does exist in a solid form and most likely has a hydrated form, CuOH × H2O.

 Please wait while we load your content...
Please wait while we load your content...