First structurally characterized self-assembly of bipodal N-thiophosphorylated bis-thiourea with CoII: magnetic properties and thermal decomposition†
Abstract
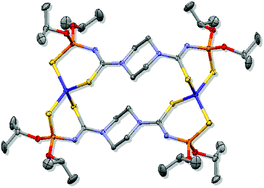
Reaction of ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S and P
S and P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S sulfur atoms. Investigation of the temperature dependence of the magnetic susceptibility is consistent with two effectively non-interacting CoII S = 3/2 ions.
S sulfur atoms. Investigation of the temperature dependence of the magnetic susceptibility is consistent with two effectively non-interacting CoII S = 3/2 ions.

 Please wait while we load your content...
Please wait while we load your content...