New insight into the role of a base in the mechanism of imine transfer hydrogenation on a Ru(ii) half-sandwich complex†
Abstract
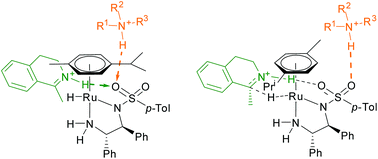
Asymmetric transfer hydrogenation (ATH) of cyclic imines using [RuCl(η6-p-cymene)TsDPEN] (TsDPEN = N-tosyl-1,2-diphenylethylenediamine) was tested with various aliphatic (secondary, tertiary) and aromatic amines employed in the HCOOH–base hydrogen donor mixture. Significant differences in reaction rates and stereoselectivity were observed, which pointed to the fact that the role of the base in the overall mechanism could be more significant than generally accepted. The hydrogenation mixture was studied by nuclear magnetic resonance (NMR), Fourier transform ion cyclotron resonance mass spectrometry (FT-ICR MS) and vibrational circular dichroism (VCD) with infrared spectroscopy. The results suggested that the protonated base formed an associate with the active ruthenium-hydride species, most probably via a hydrogen bond with the sulfonyl group of the complex. It is assumed that the steric and electronic differences among the bases were responsible for the results of the initial ATH experiments.

 Please wait while we load your content...
Please wait while we load your content...