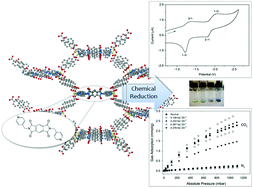
A new microporous framework, Zn(NDC)(DPMBI) (where NDC = 2,7-naphthalene dicarboxylate and DPMBI = N,N′-di-(4-pyridylmethyl)-1,2,4,5-benzenetetracarboxydiimide), containing the redox-active benzenetetracarboxydiimide (also known as pyromellitic diimide) ligand core has been crystallographically characterised and exhibits a BET surface area of 608.2 ± 0.7 m2 g−1. The crystallinity of the material is retained upon chemical reduction with sodium naphthalenide (NaNp), which generates the monoradical anion of the pyromellitic diimide ligand in the framework Zn(NDC)(DPMBI)·Nax (where x represents the molar Na+/Zn2+ ratio of 0.109, 0.233, 0.367 and 0.378 from ICP-AES), as determined by EPR, solid state Vis-NIR spectroelectrochemistry and UV-Vis-NIR spectroscopy. The CO2 uptake in the reduced materials relative to the neutral framework is enhanced up to a Na+/Zn2+ molar ratio of 0.367; however, beyond this concentration the surface area and CO2 uptake decrease due to pore obstruction. The CO2 isosteric heat of adsorption (|Qst|) and CO2/N2 selectivity (S), obtained from pure gas adsorption isotherms and Ideal Adsorbed Solution Theory (IAST) calculations, are also maximised relative to the neutral framework at this concentration of the alkali metal counter-ion. The observed enhancement in the CO2 uptake, selectivity and isoteric heat of adsorption has been attributed to stronger interactions between CO2 and both the radical DPMBI ligand backbone and the occluded Na+ ions.

 Please wait while we load your content...
Please wait while we load your content...