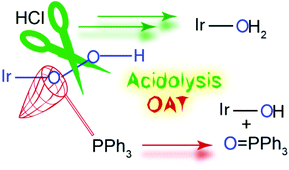
Oxygenation of Ir2II,II(tfepma)2(CNtBu)2Cl3H (1, tfepma = CH3N[P(OCH2CF3)2]2) furnishes Ir2II,II(tfepma)2(CNtBu)2Cl3(OOH) (2), the first isolable iridium hydroperoxo complex. This complex transfers an oxygen atom to triphenylphosphine, producing triphenylphosphine oxide and Ir2II,II(tfepma)2(CNtBu)2Cl3(OH) (3) in high yield. Reaction of 2 with acid induces cleavage of the O–O bond, initially forming [Ir2II,II(tfepma)2(CNtBu)2Cl3(OH2)]Cl (4), which was identified by NMR spectroscopy. With HCl as the acid source, the chloride anion displaces water to form the pseudo-C2h isomer of Ir2II,II(tfepma)2(CNtBu)2Cl4 (5). With 2,6-lutidinium chloride as the acid, complex 4 forms initially and is deprotonated by the conjugate base to afford 3 as the major product.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...