Abstract
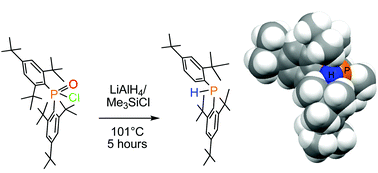
Reaction chemistry of an extremely sterically encumbered phosphinic chloride (Mes*)2P(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)Cl (Mes* = 2,4,6-tri-t-butylphenyl, supermesityl) was investigated. This compound, as well as other compounds bearing two supermesityl groups placed geminally at the central phosphorus atom, shows extremely low reactivity at the phosphorus centre. Nevertheless, some synthetically significant transformations were possible.
O)Cl (Mes* = 2,4,6-tri-t-butylphenyl, supermesityl) was investigated. This compound, as well as other compounds bearing two supermesityl groups placed geminally at the central phosphorus atom, shows extremely low reactivity at the phosphorus centre. Nevertheless, some synthetically significant transformations were possible. ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)H and a
O)H and a ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)H gave the corresponding phosphinite, which afforded very crowded
O)H gave the corresponding phosphinite, which afforded very crowded ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)R (R = Me and Et) on reactions with electrophiles. While the reaction of the phosphine Mes*(2,4-tBu2C6H3)PH with sulfur was surprisingly facile (although under forcing conditions), we have been unable to
O)R (R = Me and Et) on reactions with electrophiles. While the reaction of the phosphine Mes*(2,4-tBu2C6H3)PH with sulfur was surprisingly facile (although under forcing conditions), we have been unable to
- This article is part of the themed collection: In Celebration of David Cole-Hamilton's Career in Chemistry

 Please wait while we load your content...
Please wait while we load your content...