A thermodynamic and kinetic study of the heterolytic activation of hydrogen by frustrated borane–amine Lewis pairs†
Abstract
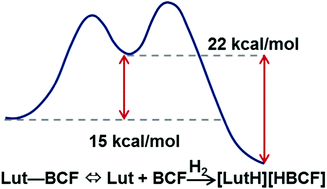
Calorimetry is used to measure the reaction enthalpies of hydrogen (H2) activation by 2,6-lutidine (Lut), 2,2,6,6-tetramethylpiperidine (TMP), N-methyl-2,2,6,6-tetramethylpiperidine (MeTMP), and tri-tert-butylphosphine (TBP) with tris(pentafluorophenyl)borane (BCF). At 6.6 bar H2 the conversion of the Lewis acid Lewis base pair to the corresponding ionic pair in bromobenzene at 294 K was quantitative in under 60 min. Integration of the heat release from the reaction of the Frustrated Lewis Pair (FLP) with H2 as a function of time yields a relative rate of hydrogenation in addition to the enthalpy of hydrogenation. The half-lives of hydrogenation range from 230 s with TMP, ΔHH2 = −31.5(0.2) kcal mol−1, to 1400 s with Lut, ΔHH2 = −23.4(0.4) kcal mol−1. The 11B nuclear magnetic resonance (NMR) spectrum of B(C6F5)3 in bromobenzene exhibits three distinct traits depending on the sterics of the Lewis base; (1) in the presence of pyridine, only the dative bond adduct pyridine–B(C6F5)3 is observed; (2) in the presence of TMP and MeTMP, only the free B(C6F5)3 is observed; and (3) in the presence of Lut, both the free B(C6F5)3 and the Lut–B(C6F5)3 adduct appear in equilibrium. A measure of the change in Keq of Lut + B(C6F5)3 ⇔ Lut–B(C6F5)3 as a function of temperature provides thermodynamic properties of the Lewis acid Lewis base adduct, ΔH = −17.9(1.0) kcal mol−1 and a ΔS = −49.2(2.5) cal mol−1 K, suggesting the Lut–B(C6F5)3 adduct is more stable in bromobenzene than in toluene.
- This article is part of the themed collection: Boranes and borohydrides

 Please wait while we load your content...
Please wait while we load your content...