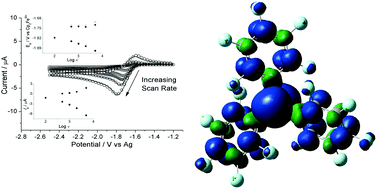
We report a kinetic and mechanistic study into the one-electron reduction of the archetypal Lewis acid tris(pentafluorophenyl)borane, B(C6F5)3, in dichloromethane and 1,2-difluorobenzene. Electrochemical experiments, combined with digital simulations, DFT computational studies and multinuclear NMR analysis allow us to obtain thermodynamic, kinetic and mechanistic information relating to the redox activity of B(C6F5)3. We show that tris(pentafluorophenyl)borane undergoes a quasi-reversible one-electron reduction followed by rapid chemical decomposition of the B(C6F5)3˙− radical anion intermediate via a solvolytic radical pathway. The reaction products form various four-coordinate borates of which [B(C6F5)4]− is a very minor product. The rate of the follow-up chemical step has a pseudo-first order rate constant of the order of 6 s−1. This value is three orders of magnitude larger than that found in previous studies performed in the donor solvent, tetrahydrofuran. The standard reduction potential of B(C6F5)3 is reported for the first time as −1.79 ± 0.1 V and −1.65 ± 0.1 V vs. ferrocene/ferrocenium in dichloromethane and 1,2-difluorobenzene respectively.
This article is Open Access
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...