A spectroscopic study on the formation of Cm(iii) acetate complexes at elevated temperatures†
Abstract
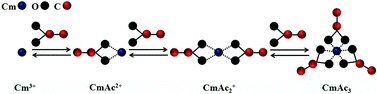
The complexation of Cm(III) with acetate is studied by time resolved laser fluorescence spectroscopy (TRLFS) as a function of ionic strength, ligand concentration, temperature and background electrolyte (NaClO4, NaCl and CaCl2 solution). The speciation of Cm(III) is determined by peak deconvolution of the emission spectra. To obtain the thermodynamic stability constants (log K0n) for the formation of [Cm(Ac)n]3−n (n = 1–3), the experimental data are extrapolated to zero ionic strength according to the specific ion interaction theory (SIT). The results show a continuous increase of the stability constants with increasing temperature (20–90 °C). The standard reaction enthalpies and entropies (ΔrH0m, ΔrS0m) of the respective reactions are derived from the integrated Van't Hoff equation. The results show that all complexation steps are endothermic and thus entropy driven (ΔrH0m and ΔrS0m > 0).

 Please wait while we load your content...
Please wait while we load your content...