A facile one-pot reduction method for the preparation of a SnO/SnO2/GNS composite for high performance lithium ion batteries†
Abstract
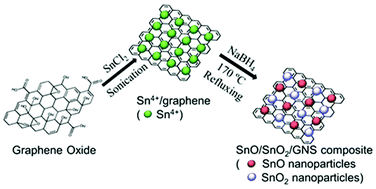
A SnO/SnO2/GNS composite with controlled oxidation states and composition has been prepared through simple one-pot reduction of an EG suspension of SnCl2 and graphene oxide. The as-prepared composite was characterized by XRD, FT-IR, XPS, SEM, TEM and BET. SnO and SnO2 nanoparticles are uniformly distributed on the surface of the graphene. Taking advantage of the high electron conductivity of graphene and the large theoretical capacity of SnO, this SnO/SnO2/GNS composite exhibits high charge/discharge capacity, good cycling stability and good rate capability. A specific discharge capacity of approximately 464.2 mA h g−1 is retained after being charged/discharged at a current density of 1000 mA g−1 for 30 cycles.

 Please wait while we load your content...
Please wait while we load your content...