An oxygen-17 dynamic NMR study of the Pr–DOTA complex†
Abstract
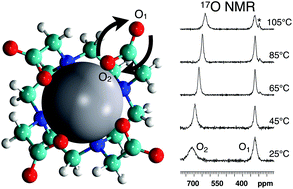
The complex between 17O-enriched DOTA (tetraazacyclododecanetetraacetic acid) and praseodymium(III) (Pr3+) was studied in aqueous solution by variable-temperature 17O NMR at 14.1 T. pH effects as well as the influence of metal ions free in solution were investigated. At low temperature, the so-called TSAP and SAP conformations give rise to distinct signals for the oxygen atoms coordinated to the metal ion (O2); coalescence occurs between 20 and 30 °C. In contrast, a single signal was detected for the noncoordinated oxygen atoms (O1) in the entire investigated temperature range, i.e. between −3 and 135 °C. At high temperature, the spectra exhibit signal broadening that reveals the interchange of the O1 and O2 oxygen atoms of the carboxylate groups. The linewidths measured for O1 were deconvolved into contributions from quadrupole relaxation and chemical exchange, allowing the corresponding activation barriers to be determined. The present 17O dynamic NMR study provides the first quantitative experimental data characterizing the interchange of the oxygen atoms in a DOTA chelate of a lanthanide metal ion. The activation entropy of this process is negligible and the activation enthalpy is found to range between 66 and 77 kJ mol−1, depending on the pH and the presence of free Pr3+ ions in solution. These data support the results of a previous computational study according to which the exchange mechanism involves the internal rotation of the carboxylate groups.

 Please wait while we load your content...
Please wait while we load your content...