Borata–alkene derivatives conveniently made by frustrated Lewis pair chemistry†
Abstract
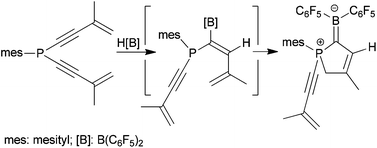
Two (aryl)PXY starting materials (aryl = mesityl or 2,4,6-triisopropylphenyl; X,Y = Cl, Br) were reacted with lithiated conjugated enynes (derived from 2-methylbutenyne or 1-ethynylcyclohexene) to yield the respective (aryl)bis(enynyl)phosphanes. Their reaction with HB(C6F5)2 gave the heterocyclic five-membered zwitterionic borata–diene compounds containing the aryl group and one unchanged enynyl substituent at phosphorus. The borata–alkene products were thought to arise from a two step process of regioselective alkyne hydroboration followed by an internal phosphane attack on the boryldiene unit. Three examples of the ring-closed borata–alkene type products were characterized by X-ray diffraction.

 Please wait while we load your content...
Please wait while we load your content...