Cationic half-sandwich quinolinophaneoxazoline-based (η6-p-cymene)ruthenium(ii) complexes exhibiting different chirality types: synthesis and structural determination by complementary spectroscopic methods†‡
Abstract
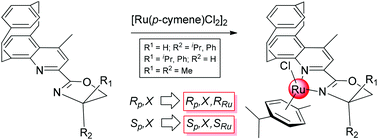
Novel, optically pure 2-{4-methyl[2]paracyclo[2](5,8)quinolinophan-2-yl}-4-aryl/alkyloxazolines (QUIPHANOX) exhibiting both planar and central chirality have been prepared by reacting (Rp)- and (Sp)-2-cyano-4-methyl[2]paracyclo[2](5,8)quinolinophane with 2-aryl/alkyl-2-aminoethanols. The reaction of each of the above N,N-ligands with [Ru(η6-p-cymene)Cl2]2 in methanol, in the presence of either NH4PF6 or NaBPh4, gave the corresponding half-sandwich [(η6-p-cymene)Ru(QUIPHANOX)Cl]+Y−, (Y− = PF6−, BPh4−) as stable complex salts exhibiting planar and carbon- and metal-centered chirality. The unknown absolute configuration (AC) at the metal was determined by 1H NMR and by theoretical calculations of electronic circular dichroism (ECD) spectra and subsequently confirmed by crystallographic X-ray analysis. (Rp) and (Sp)-ligands afforded (RRu)- and (SRu)-configured complexes, respectively, independent of the AC at the chiral carbon of the oxazoline moiety.

 Please wait while we load your content...
Please wait while we load your content...