Chiroptical properties, binding affinity, and photostability of a conjugated zinc porphyrin dimer complexed with left-handed Z-DNA and right-handed B-DNA†
Abstract
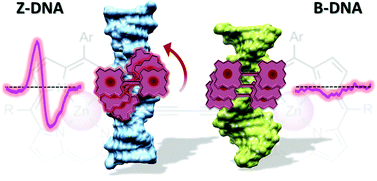
We have studied the UV-vis absorption and chiroptical properties, binding affinity and photostability of a conjugated positively charged butadiyne-linked Zn(II) porphyrin dimer bound to DNA sequence poly(dG-dC)2. Right-handed B-DNA, spermine-induced Z-DNA and Co(III)-induced Z-DNA have been explored. Resonance light scattering (RLS) spectra showed formation of porphyrin aggregates in the presence of all DNA forms with the largest aggregates formed with B-DNA. The porphyrin dimer gave rise to induced bisignate circular dichroism (CD) signals in the presence of the left-handed Z-DNA conformations. On the other hand, the dimer stayed nearly chiroptically silent when complexed with the B-form of poly(dG-dC)2. Our results indicated that the conjugated Zn(II) porphyrin dimer can be used as a sensor for the chiroptical detection of Z-DNA in the visible (400–500 nm) and near-infrared region of the electromagnetic spectrum (700–800 nm). The helicity of DNA had little effect on the dimer binding affinities. The photostability of the porphyrin dimer complexed with any form of DNA was higher than that of the free molecule. The porphyrin dimer bound to Z-DNA exhibited slower photobleaching than the B-DNA dimer complex.

 Please wait while we load your content...
Please wait while we load your content...