Phosphine-substituted sterically encumbered pyrrolyl ligands – synthesis and reactivity studies†
Abstract
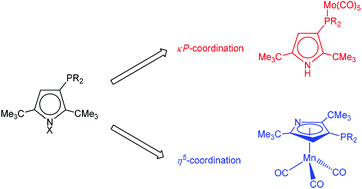
The sterically encumbered pyrrolyl KPyrtBu2 (1-K, PyrtBu2 = 2,5-(Me3C)2C4H2N) reacts with PCl3, (iPr)2PCl and Ph2PCl exclusively at the C3-position to yield a series of phosphine-substituted pyrroles HPyrtBu2R (R = PCl2 (2-H), (iPr)2P (4-H) and Ph2P (5-H)). Pyrrole 2-H can further be functionalized with MeLi (3 equiv.) to yield LiPyrtBu2PMe2 (3-Li). The coordination chemistry of these phosphine-substituted pyrroles was explored and the resulting complexes were characterized by various spectroscopic techniques and in some cases using single crystal X-ray diffraction. The neutral pyrroles 4-H and 5-H react cleanly with [Mo(CO)6] to form the κP-coordinate complexes 4-H–Mo(CO)5 and 5-H–Mo(CO)5, respectively. IR spectroscopy of these complexes shows that C3-substitution barely changes the π-acceptor properties of the phosphine moiety. The η5-coordination was achieved when pyrrolides 4-K and 5-K were reacted with [Mn(CO)5Br] to give 4-Mn(CO)3 and 5-Mn(CO)3, respectively. The energy of the HOMOs in these three legged-piano stool complexes decreases on PPh2-substitution (5-Mn(CO)3) stabilizing the Mn–CO bonds, whereas the inverse effect is noticed for P(iPr)2-substitution (4-Mn(CO)3).

 Please wait while we load your content...
Please wait while we load your content...