A structural study of a three-membered linear metal chain compound at elevated pressure†
Abstract
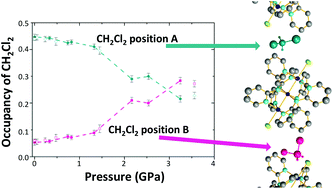
Single crystal X-ray diffraction studies of the symmetrical molecular wire compound Co3(dpa)4Cl2·(dcm) have been performed up to a pressure of 3.6 GPa using both synchrotron and conventional sources. It is found that the terminal Co–Cl bond distance initially increases by 0.013(4) Å at 0.32 GPa after which it continuously decreases. Extensive theoretical calculations show that population of a thermally excited state containing increased Co–Cl anti-bonding character is a possibility at 0.32 GPa. The relative occupancy of the disordered dcm solvent molecule changes significantly with pressure and this is explained by the analysis of void spaces and Hirshfeld surfaces at different pressures. At 3.2 GPa, fingerprint plots derived from Hirshfeld surfaces indicate that neighbouring metal chain compounds approach each other such that short H⋯H interactions appear.

 Please wait while we load your content...
Please wait while we load your content...